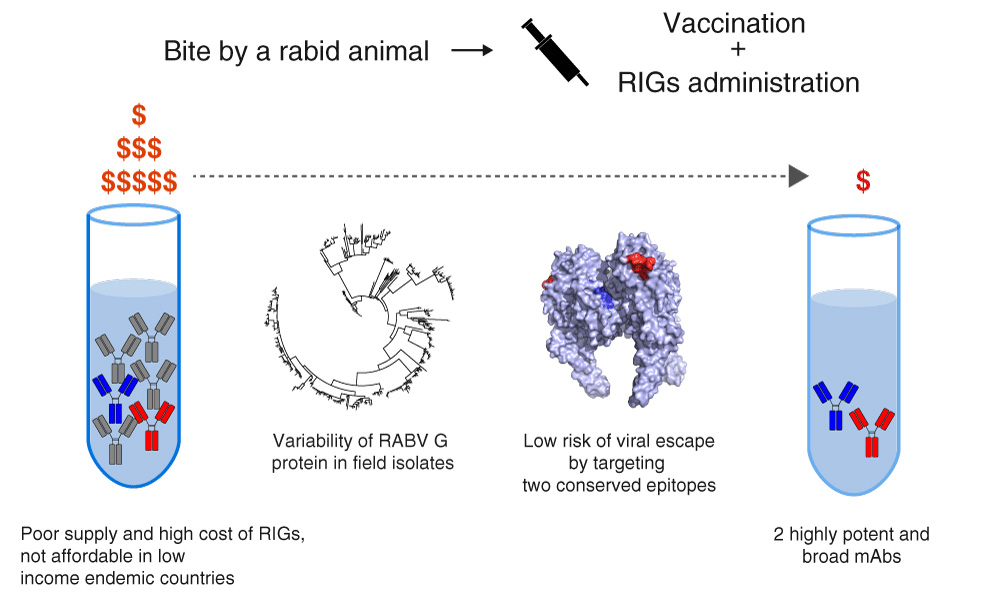
The difficulty to access a low-cost hyperimmune treatment which may satisfy the global demand has led to the development of a monoclonal antibody cocktail. Poor and under developed endemic countries will mostly benefit from its production and distribution and we hope this health burden, which causes a high but unnecessary yearly human cost, may be drastically reduced.
Two monoclonal antibodies with broad-spectrum and high potency against rabies viruses and rabies-related lyssaviruses have been identified and characterized.
The results of the research undertaken by the Istituto Zooprofilattico Sperimentale delle Venezie (IZSVe) based in Padua, in an international collaboration with leading research institutes from Switzerland, France and the UK and published in this week’s EMBO Molecular Medicine, demonstrate that the RVC20 and RVC58 monoclonal antibody cocktail, obtained from a human vaccinated donor, is 1,000 times more effective than existing treatments based on human immunoglobulins. RVC20 and RVC58 may provide a very effective and affordable alternative to existing post-exposure prophylaxis of rabies infection.
Some 59,000 people are estimated to die each year, mainly in India, China and Africa, and 50% of rabies cases worldwide affect children. However, the true burden of rabies in developing countries is unknown and largely undiagnosed.
Rabies is found worldwide in different animal reservoirs and is known to be a deadly viral infection with almost 100% fatality following the onset of symptoms. No treatment is presently available. However, the time interval for infected humans to be treated by vaccination and co-administration of rabies immunoglobulins prepared from human or equine blood, before the onset of symptoms is very short. This so-called post-exposure prophylaxis (PEP) must be administered as early as possible and is ineffective after the onset of symptoms.
Paola De Benedictis, lead author of the paper and virologist and veterinarian at IZSVe (FAO reference centre for rabies, OIE collaborating centre for diseases at the animal-human interface) said:
“The antibody cocktail that we developed has great potential. It may not only replace immunoglobulins but it can also pave the way to a paradigm shift in rabies treatment. We noticed that in vivo even 40 days after administration of the antibodies, there still seemed to be a high neutralizing and potentially protective titer of the antibodies in the blood, so it may be that the prophylaxis window has widened considerably more than expected by using RVC20 and RVC58.”
The high cost and limited availability of PEP is a huge problem, especially in remote areas in the poor parts of the world. Only about 1 million doses of rabies immunoglobulins are produced every year and sadly 60% of the people at high risk of developing rabies do not have access to them.
The transition from RIG to monoclonal antibody-based PEP is therefore strongly recommended by the WHO, whose aim is that of being provided with an adequate supply at affordable costs.
Davide Corti, co-author of the paper and CSO of Humabs BioMed (Bellinzona, Switzerland) said:
“Our development is in line with recommendations by the WHO to develop new, monoclonal antibody-based treatments for rabies post-exposure prophylaxis. The antibody cocktail we identified and tested has high potency, targets distinct, non-overlapping antigens and broadly covers all known viruses. Moreover, the high potency of these antibodies demonstrated that only a very limited amount, as low as 3 milligrams per dose, is needed to protect humans from this lethal infection.”
Hervé Bourhy, co-author of the paper and Head of the WHO Collaborating Centre for Reference and Research on Rabies at the Institute Pasteur (Paris, France) said:
“Current post-exposure prophylaxis is very costly and supply is dwindling because of lack of human donors. Alternatives under development so far protect only against a limited spectrum of viruses, posing serious concerns for prophylaxis without virus characterization. The antibodies we characterized in vitro protect against a very broad spectrum of rabies viruses. Moreover, these antibodies could be formulated in ways that do not require cooling, another important advantage in remote areas.”
Filippo Riva, CEO of Humabs BioMed, added:
“The isolation of these two antibodies is another validation of our approach that can lead to very potent antibodies against deadly infectious diseases. Within the past six months, we have demonstrated this in MERS, Ebola and now in Rabies. We are now seeking licensing opportunities on a worldwide basis and we are more than positive that the development of these antibodies may start as soon as possible.”
Further information
- Read the full scientific article – EMBO Molecular Medicine
- FAO reference centre for rabies
- OIE collaborating centre for diseases at the human/animal interface
- Humabs BioMed
- Institute Pasteur – Laboratory Lyssavirus Dynamics and Host Adaptation
- Institute for Research in Biomedicine
- University of Westminster